Which of These Molecules Is Nonpolar
Alkynes are considered nonpolar molecules because they dont dissolve in water. Carbon monoxide is a linear molecule but the electronegativity difference between carbon and oxygen is significant enough to make the molecule polar.
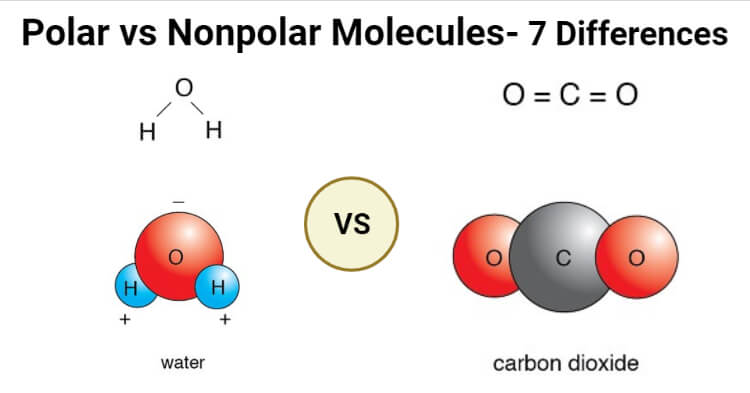
Polar Vs Nonpolar Molecules Definition 7 Key Differences Examples
H 2 N 2 O 2 Cl 2 These are truly nonpolar molecules Carbon dioxide - CO 2.

. Electrons are equally shared. The most common nonpolar molecules are hydrocarbons. Non Polar molecules are those molecules in which share electrons equally in a covalent bond.
A notable exception is carbon monoxide CO. Most carbon compounds are nonpolar. Covalent molecules that have atoms with the same electronegativity values.
Hence the molecule is non polar with zero dipole moment. Titrate nonpolar and polar substances. This queston does not provides the molecules that are needed to be checked in order to classify them as polar or non-polar.
Any of the homonuclear diatomic element like H2 N2 O2 Cl2 etc. The individual bond dipoles cancel each other as they point in opposite direction and are equal in magnitude. Act as a catalyst in a reaction between 2 polar molecules.
Hydrocarbon liquids such as gasoline and toluene. The geometry of co2 is linear which makes the dipole moments of both its bonds cancel out. A sharing of electron pairs B attraction between polar molecules C bonding of a covalent bonded hydrogen to an unshared electron pair D more attraction between ions E more than one A Which of the following covalent bonds is the most polar.
Area with a higher electronegativity value closer to F region. Polar bonds- water carbon dioxide. The molecule has linear geometry hence the.
NON POLAR MOLECULES. Carbon tetrachloride - CCl 4. Form covalent bonds with water molecules.
Carbon and hydrogen have relatively equal electronegativity and so the electrons are shared equally and the molecule is nonpolar. These are molecules made entirely of carbon and hydrogen atoms. Carbon dioxide is the molecule having polar bonds but the molecule is itself a non-polar.
Learn vocabulary terms and more with flashcards games and other study tools. Which of these molecules is polar. BeCl 2 or Beryllium Dichloride is a nonpolar molecule as there is zero net dipole moment.
CO2 is non-polar while SO2 is polar in nature. Water alcohol Sulphur dioxide ammonia ethanol hydrogen sulfide bent molecules those with a significant bond angle in general. - 18691711 A 200-L insulated tank contains nitrogen gas at 200 kPa 300 K.
Electrons are not equally shared. The molecule has linear geometry hence the dipole moments in the molecule get nullified. Carbon dioxide - CO2.
Start studying Are these molecules polar or nonpolar. Some examples of nonpolar molecules include noble gases carbon dioxide methane benzene homo-nuclear diatomic molecules oxygen propane butane sulfate carbon tetrachloride ethylene hydrocarbons toluene gasoline sulfur hexafluoride beryllium dichloride acetylene fats turpentine and alkanes. Since electrons are shared equally therefore there is no net electrical charge across the molecule.
Carbon dioxide C O 2 has polar bonds but is a nonpolar molecule. Any of the noble gasses. Similarly oxygen gas is made of two oxygen atoms.
Area with lower electronegativity value. Has dipoles and - regions - region. He Ne Ar Kr Xe These are atoms not technically molecules Any of the homonuclear diatomic elements.
SHOW MORE a All polar molecules have at least one polar bond b All non polar molecules have non polar bonds с The higher the electronegativity of an atom the more polar the atom is d The higher electronegativity difference between. Since carbon dioxide makes a linear shape therefore the polarity from the opposites oxygen atoms cancels out and the molecule becomes non-polar. Nonpolar bonds- ozone oxygen.
The structure of C O 2 is linear. Nonpolar molecules have no net dipoles. Methane - CH 4.
The geometry of so2 is bent which means that the dipole moments of each bond. Cause polar and nonpolar molecules to mix. Ethylene - C 2 H 4.
Benzene - C 6 H 6. Hydrocarbons gasoline toluene homo-nuclear diatomic molecules O 2 N 2 Cl 2 H 2 etc noble gases benzene methane ethylene carbon tetrachloride. A line with nitrogen at 500 K 500 kPa adds 40 more mass to the tank with a flow thro.
What do Nonpolar molecules have.

Image Result For Polar Vs Nonpolar Molecules Covalent Bonding Chemical Bond Chemistry Lessons
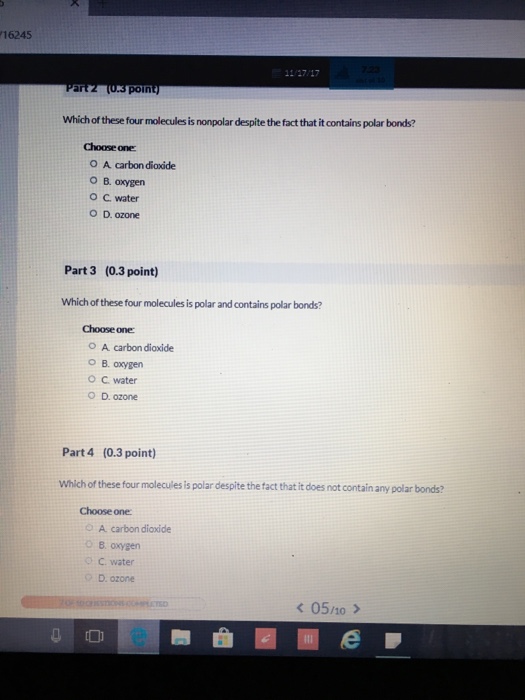
Solved 6245 Poin Which Of These Four Molecules Is Nonpolar Chegg Com
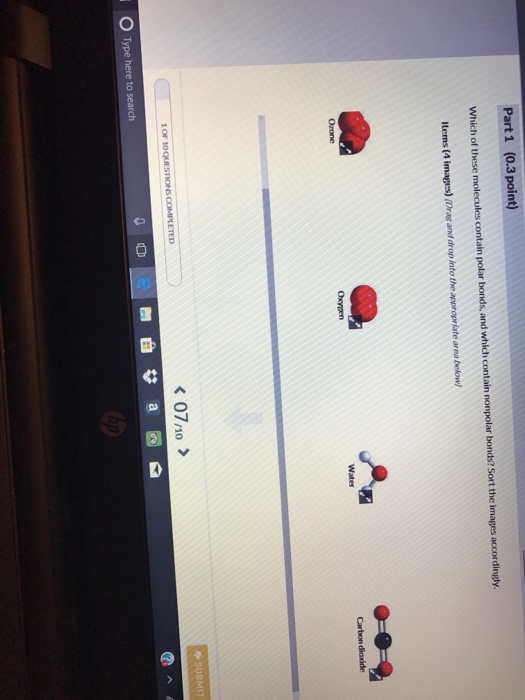
Solved Part 1 0 3 Point Which Of These Molecules Contain Chegg Com
Comments
Post a Comment